May 2025 Patent Highlights: LP(a) inhibitor from CSPC is better?
Last year, AstraZeneca entered into an exclusive licensing agreement with CSPC Pharmaceutical Group Co., Ltd. (CSPC) to advance the development of an early stage,novel small molecule lipoprotein(a) (Lp(a)) disruptor that has the potential to offer additional benefits for patients with dyslipidaemia. Under the terms of the agreement, AstraZeneca will receive access to CSPC’s pre-clinical candidate small molecule, YS2302018, an oral Lp(a) disruptor, with the aim of developing this as a novel lipid-lowering therapy with potential in a range of cardiovascular disease indications alone or in combination, including with the oral small molecule PCSK9 inhibitor, AZD0780.
YS2302018 was discovered by CSPC and has been shown to effectively prevent the formation of Lp(a). The structure of YS2302018 has not yet been disclosed, but it may be related to the compounds claimed in these new patent applications. The example in WO2025103442A1 applied by Innovstone therapeutics limited, a subsidiary of CSPC, has passed the cell biology and animal model pharmacodynamics evaluation, showing higher Lp(a) inhibition results compared to Eli Lilly’s LY3473329.
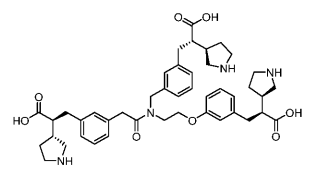
Compound 96(WO2025103442)
Lp(a)/IC50(nM):0.58
Lp(a) inhibition rate(5mg/kg):95%
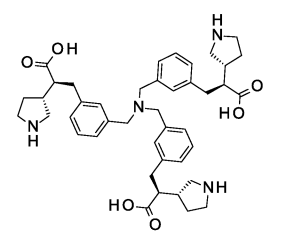
Muvalaplin (LY3473329)
Lp(a)/IC50(nM):0.09(ESC2023)
Lp(a) inhibition rate(5mg/kg):56%(WO2025103442)
In terms of safety, the information disclosed in the patent shows that the compound or LY3473329 (300 mg/kg, 1000 mg/kg) was administered once a day for 14 consecutive days. No changes related to the compound or LY3473329 were found in the clinical observations, body weight, food intake, hematology, blood biochemistry, urine, organ weight and tissue pathology of all animals.
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!