Request Demo
Last update 23 Aug 2025
Polatuzumab
Last update 23 Aug 2025
Overview
Basic Info
Drug Type Monoclonal antibody |
Synonyms- |
Target |
Action inhibitors |
Mechanism CD79B inhibitors(B-cell antigen receptor complex-associated protein beta chain inhibitors) |
Therapeutic Areas |
Active Indication |
Inactive Indication- |
Originator Organization |
Active Organization |
Inactive Organization- |
License Organization- |
Drug Highest PhaseClinical |
First Approval Date- |
Regulation- |
Structure/Sequence
Sequence Code 31655L

The sequence is quoted from: *****
Sequence Code 9976257H

The sequence is quoted from: *****
Related
100 Clinical Results associated with Polatuzumab
Login to view more data
100 Translational Medicine associated with Polatuzumab
Login to view more data
100 Patents (Medical) associated with Polatuzumab
Login to view more data
59
Literatures (Medical) associated with Polatuzumab01 Jul 2025·Blood Cancer Discovery
B-cell Receptor Silencing Reveals the Origin and Dependencies of High-Grade B-cell Lymphomas with MYC and BCL2 Rearrangements
Article
Author: Pietrini, Ilaria ; Capaccio, Pasquale ; Pellegrini, Vilma ; Balzarini, Piera ; Hohaus, Stefan ; Ungari, Marco ; Vit, Filippo ; Giampaolo, Sabrina ; Varasi, Mario ; Sivacegaram, Anojan ; Di Napoli, Arianna ; Gonzalez, Cristina Lopez ; Ferreri, Andres J.M. ; Bertolazzi, Giorgio ; Mercurio, Ciro ; Pizzi, Marco ; Thanasi, Hajdica ; Bugatti, Mattia ; Falini, Brunangelo ; Bomben, Riccardo ; Li, Ying ; Lorenzi, Luisa ; Varano, Gabriele ; Lorenzini, Daniele ; Albertini, Emma ; Cicio, Giada ; Tripodo, Claudio ; Ranise, Cecilia ; Morlacchi, Elena ; Trifiro, Paolo ; Pagani, Chiara ; Robusto, Michela ; Pruneri, Giancarlo ; Selvarasa, Viveka ; Leuzzi, Brian ; Maiolo, Elena ; Morello, Gaia ; Facchetti, Fabio ; Segura-Garzon, Daniel ; Andronache, Adrian ; Tucci, Alessandra ; Mehr, Ramit ; Larocca, Luigi M. ; Almici, Camillo ; Taddei, Tommaso M. ; Cabras, Antonello D. ; Chiarini, Marco ; Yang, Henry ; Cancila, Valeria ; Casola, Stefano ; Chan, Wing C. ; Minardi, Simone P. ; Arima, Hiroshi ; Neuman, Hadas ; Sindaco, Paola ; Pignataro, Lorenzo ; Sonoki, Takashi ; Riboni, Mirko ; Parr, Nicara C. ; Visco, Euplio ; Song, Joo Y. ; Siebert, Reiner ; Brambillasca, Silvia ; Lonardi, Silvia ; Mainoldi, Federica ; Bonnal, Raoul J.P. ; Daffini, Rosa ; Bellesi, Silvia ; Torretta, Sara ; Ponzoni, Maurilio
Abstract:
The B-cell receptor (BCR) is critical for mature B-cell lymphomas (BCL), serving as a therapeutic target. We show that high-grade BCLs with MYC and BCL2 rearrangements [HGBCL–double-hit (DH)–BCL2] predominantly exhibit immunoglobulin heavy (IGH) chain silencing, leading to BCR shutdown. IGH-silenced HGBCL-DH-BCL2 (IGHUND) tumors differ from IGH+ counterparts in germinal center (GC) zone programs, MYC expression, and immune infiltrate. Whereas IGH+ HGBCL-DH-BCL2 tumors favor IGM/IG-κ expression, IGHUND counterparts complete IGH isotype switching and IG-λ rearrangements. IGHUND lymphomas retain productive IGHV rearrangements and require IGH for optimal fitness. BCR silencing, caused by accelerated IGH turnover and reduced IGH expression, precedes HGBCL-DH-BCL2 onset, inducing RAG1/2-dependent IG light chain editing and facilitating t(8;22)/IGL::MYC translocations. IGHUND HGBCL-DH-BCL2 models exhibit reduced sensitivity to the CD79B-targeting antibody–drug conjugate polatuzumab vedotin. Collectively, HGBCL-DH-BCL2 commonly arises from isotype-switched t(14;18)+ GC B cells, which edit IG light chains, fueling intraclonal diversification, BCR extinction, and t(8;22) while maintaining IGH dependence, with clinical implications.
Significance::
These findings link BCR silencing in IGH isotype-switched t(14;18)+ GC B cells to RAG1/2 expression, which triggers IG light chain editing and predisposes to IGL::MYC translocations, promoting HGBCL. In HGBCL with MYC and BCL2 rearrangements, BCR silencing protects from polatuzumab vedotin killing.See related commentary by Shevchenko and Hodson, p. 284
04 Jun 2025·MOLECULAR CANCER THERAPEUTICS
Broadening the Therapeutic Window of ADCs Using Site-Specific Bioconjugation Showcased by an MMAE-Containing Peptide Linker in a CD79b-Targeting ADC
Article
Author: Stark, Ramona ; Probst, Philipp ; Grabulovski, Dragan ; Schlereth, Bernd ; Bertrand, Romain ; Attinger-Toller, Isabella ; Santimaria, Roger ; Spycher, Philipp René
Abstract:
The limitations of first-generation antibody–drug conjugate (ADC) technologies include suboptimal stability and efficacy, poor safety profiles, and challenging manufacturing processes. In this study, we describe an anti–CD79b-monomethyl auristatin E (MMAE) ADC generated using a novel peptide-based linker technology that allows for site-specific linker-payload conjugation to native antibodies in only one step. The ADC comprises native polatuzumab as the targeting antibody and a linker-payload consisting of a RKAA-peptide linker and MMAE. We compared our anti–CD79b-RKAA-MMAE ADC with polatuzumab vedotin (PV), the FDA-approved ADC for diffuse large B-cell lymphoma. In the clinic, PV shows significant instability in circulation, leading to strong and dose-limiting side effects, including neutropenia and peripheral neuropathy. The anti–CD79b-RKAA-MMAE ADC showed optimal biophysical properties with a well-defined drug-to-antibody ratio of 2. It demonstrated potent cytotoxicity in multiple cancer cell lines and was very stable in mouse, cynomolgus monkey, and human sera. The anti–CD79b-RKAA-MMAE conjugate showed equal antitumor efficacy at half the payload dose compared with PV in different xenograft models. At equal MMAE concentrations, greater tumor growth inhibition and a considerably longer duration of response were observed. Ultimately, the highest nonseverely toxic dose of 30 mg/kg was determined in a 4-week repeat-dose toxicology study in rats, which is a 3-fold higher ADC dose than reported for PV. In summary, the data show that our novel site-specific bioconjugation technology enabled the generation of an anti–CD79b-RKAA-MMAE ADC with highly favorable biophysical properties and a greatly improved therapeutic index by a factor of 4 to 6 compared with PV. The ADC may therefore represent a safe and efficacious alternative for patients with diffuse large B-cell lymphoma.
01 Jun 2025·Clinical Lymphoma Myeloma & Leukemia
SOHO State of the Art Updates and Next Questions | Diffuse Large B-Cell Lymphoma in Older Adults: A Comprehensive Review
Review
Author: Iyengar, Varun ; Hamlin, Paul ; Torka, Pallawi
Older adults (OA) with DLBCL are a heterogenous population with suboptimal outcomes. In this review, we identify and address the unique challenges encountered in the care of OA with DLBCL. We elaborate on the role and limitations of current geriatric assessment (GA) tools and ways to incorporate fitness in therapeutic decision making. We suggest best practices to implement GA in routine practice and clinical trials. The most widely used tool is simplified GA (sGA) which categorizes patients into fit, unfit and frail groups. Patients who are fit benefit from full dose/curative approach, whereas consideration should be made to reduce the intensity of chemotherapy for unfit patients. Frail patients with DLBCL are a major unmet need without any satisfactory treatment options. Ongoing investigations combining novel therapies into chemotherapy-free regimens are underway with promising early results. In the relapsed/refractory (R/R) setting, anti-CD19 CAR-T cell therapy (CART) is now the standard of care for primary refractory disease or relapse within 12 months of completing therapy. Autologous stem cell transplant is still a consideration for fit OA with relapse >12 months after completing therapy. The recent approval of bispecific antibodies is a welcome advance that will greatly benefit OA not eligible for CART. Other regimens available for patients ineligible for CART or for those who experience progression post-CART include polatuzumab-rituximab±bendamustine, tafasitamab-lenalidomide, loncastuximab or chemotherapy-based approaches such as rituximab-gemcitabine-oxaliplatin. We discuss the changing paradigm in R/R DLBCL and spotlight emerging data from recent congresses that can improve outcomes in this vulnerable population.
5
News (Medical) associated with Polatuzumab14 May 2025
Media ReleaseCOPENHAGEN, Denmark; May 14, 2025 Data from 14 abstracts highlight the depth, breadth, and strength of Genmab’s comprehensive epcoritamab development program across multiple patient populations and treatment settings Genmab A/S (Nasdaq: GMAB) announced today that it will present 14 abstracts evaluating epcoritamab, a T-cell engaging bispecific antibody administered subcutaneously, as a monotherapy and in combination across disease settings in patients with diffuse large B-cell lymphoma (DLBCL) and follicular lymphoma (FL) at the 30th European Hematology Association (EHA) Congress, being held in Milan, Italy, and virtually, June 12-15, 2025. Two oral presentations will feature data from the Phase 1/2 EPCORE® NHL-2 trial evaluating epcoritamab plus rituximab and ifosfamide-carboplatin-etoposide (R-ICE) in patients with relapsed/refractory (R/R) DLBCL eligible for autologous stem cell transplantation, and the Phase 1/2 EPCORE NHL-5 trial evaluating epcoritamab plus polatuzumab vedotin, rituximab, cyclophosphamide, doxorubicin, and prednisone (pola-R-CHP) in previously-untreated patients with DLBCL. Additionally, results from the Phase 1/2 EPCORE NHL-1 and NHL-3 trials, including three years of follow-up in patients with R/R DLBCL and FL treated with epcoritamab monotherapy, will be presented as a poster. All abstracts accepted for presentation have been published and may be accessed online via the EHA Open Access Library. “Together with AbbVie, we have made tremendous progress advancing our broad epcoritamab development program and we are pleased to share important results at EHA 2025 evaluating epcoritamab in a variety of treatment settings and patient populations,” said Dr. Judith Klimovsky, Executive Vice President and Chief Development Officer of Genmab. “The data presented at EHA further reinforce our commitment to epcoritamab and its potential to become a core therapy across B-cell malignancies.” Several abstracts evaluating epcoritamab will also be presented at the 18th International Conference on Malignant Lymphoma (ICML), taking place June 17-21, 2025, in Lugano, Switzerland. Abstracts accepted for presentation at EHA include: Abstract Number Abstract Title Type of Presentation Date/Time of Presentation S245First Disclosure of Epcoritamab + R-ICE in Patients with Relapsed/Refractory Diffuse Large B-cell Lymphoma (R/R DLBCL) Eligible for Autologous Stem Cell Transplantation (ASCT): EPCORE NHL-2OralSunday, June 1511:00-12:15 CESTS247Durable Efficacy with Fixed-Duration Epcoritamab + Polatuzumab Vedotin, Rituximab, Cyclophosphamide, Doxorubicin, and Prednisone (Pola-R-CHP) for 1L Diffuse Large B-cell Lymphoma (EPCORE NHL-5)OralSunday, June 1511:00-12:15 CESTPF881Epcoritamab Monotherapy Demonstrates Deep and Durable Responses at Three-Year follow-up in Patients with Relapsed/Refractory Follicular LymphomaPosterFriday, June 13 18:30-19:30 CESTPF885Epcoritamab Plus Lenalidomide and Rituximab Achieves High Response Rates and Survival Benefits Compared with Usual Care in Relapsed/Refractory Follicular Lymphoma: A Comparative AnalysisPosterFriday, June 13 18:30-19:30 CESTPF920Sustained Remission in R/R DLBCL with Epcoritamab Monotherapy: EPCORE NHL-1 3y Results and Novel Subgroup Analyses in Patients with Complete Response at 2yPosterFriday, June 13 18:30-19:30 CESTPS1886Matching-Adjusted Indirect Comparison of Epcoritamab with Rituximab + Lenalidomide vs Tafasitamab with Rituximab + Lenalidomide in Second-Line+ Follicular LymphomaPosterSaturday, June 14 18:30-19:30 CESTPS1898Patient-Reported Outcomes in Patients with Relapsed/Refractory Follicular Lymphoma Treated with Epcoritamab within the Entire Study Cohort and in Patients with Symptoms at BaselinePosterSaturday, June 14 18:30-19:30 CESTPS1968Match-Adjusted Comparative Analysis of Epcoritamab + R-DHAX/C or R-ICE vs R-DHAX/C or R-ICE In 2L+ Transplant-Eligible Patients with Diffuse Large B-Cell LymphomaPosterSaturday, June 14 18:30-19:30 CESTPS1942Match-Adjusted Comparative Analysis of the Efficacy Of Epcoritamab + R-Mini-CHOP vs R-Mini-CHOP in Previously Untreated Diffuse Large B-Cell LymphomaPosterSaturday, June 14 18:30-19:30 CESTPS1932Treatment Outcomes in Newly Diagnosed Diffuse Large B-Cell Lymphoma Patients with High Cardiovascular Risk, and Treated with Non-Anthracycline Containing RegimensPosterSaturday, June 14 18:30-19:30 CESTPS1944Patient Preferences for Attributes of Bispecific Antibodies Indicated for the Treatment of Relapsed/Refractory Diffuse Large B-Cell Lymphoma in the United StatesPosterSaturday, June 14 18:30-19:30 CESTPS1912Circulating Tumour DNA-Directed Intervention with Epcoritamab Alone or in Combination with Lenalidomide and Rituximab is Feasible in the Early Post-CAR-T Population at High Risk of Relapse: Preliminary Data from EpLCARTPosterSaturday, June 14 18:30-19:30 CESTPS1979Epcoritamab with Gemcitabine, Dexamethasone, and Cisplatin (Epco-GDP) in Relapsed, Refractory Large B-cell Lymphoma – An Interim Analysis of Phase II Multicenter Investigator-initiated TrialPosterSaturday, June 1415:30-16:00 CESTPS1892Phase II Investigator-initiated Trial of Epcoritamab-Lenalidomide in Treatment Naïve Follicular LymphomaPosterSaturday, June 14 18:30-19:30 CEST The safety and efficacy of these investigational uses have not been established. About Epcoritamab Epcoritamab is an IgG1-bispecific antibody created using Genmab's proprietary DuoBody® technology and administered subcutaneously. Genmab's DuoBody-CD3 technology is designed to direct cytotoxic T cells selectively to elicit an immune response toward target cell types. Epcoritamab is designed to simultaneously bind to CD3 on T cells and CD20 on B cells and induces T-cell-mediated killing of CD20+ cells.i Epcoritamab (approved under the brand name EPKINLY® in the U.S. and Japan, and TEPKINLY® in the EU) has received regulatory approval in certain lymphoma indications in several territories. Epcoritamab is being co-developed by Genmab and AbbVie as part of the companies' oncology collaboration. The companies will share commercial responsibilities in the U.S. and Japan, with AbbVie responsible for further global commercialization. Both companies will pursue additional international regulatory approvals for the investigational R/R FL indication and additional approvals for the R/R DLBCL indication. Genmab and AbbVie continue to evaluate the use of epcoritamab as a monotherapy, and in combination, across lines of therapy in a range of hematologic malignancies. This includes five ongoing Phase 3, open-label, randomized trials including a trial evaluating epcoritamab as a monotherapy in patients with R/R DLBCL compared to investigators choice chemotherapy (NCT04628494), a trial evaluating epcoritamab in combination with R-CHOP in adult patients with newly diagnosed DLBCL (NCT05578976), a trial evaluating epcoritamab in combination with rituximab and lenalidomide (R2) in patients with R/R FL (NCT05409066), a trial evaluating epcoritamab in combination with rituximab and lenalidomide (R2) compared to chemoimmunotherapy in patients with previously untreated FL (NCT06191744), and a trial evaluating epcoritamab in combination with lenalidomide compared to chemoimmunotherapy in patients with R/R DLBCL (NCT06508658). The safety and efficacy of epcoritamab has not been established for these investigational uses. Please visit www.clinicaltrials.gov for more information. About Genmab Genmab is an international biotechnology company with a core purpose of guiding its unstoppable team to strive toward improving the lives of patients with innovative and differentiated antibody therapeutics. For more than 25 years, its passionate, innovative and collaborative team has invented next-generation antibody technology platforms and leveraged translational, quantitative and data sciences, resulting in a proprietary pipeline including bispecific T-cell engagers, antibody-drug conjugates, next-generation immune checkpoint modulators and effector function-enhanced antibodies. By 2030, Genmab’s vision is to transform the lives of people with cancer and other serious diseases with knock-your-socks-off (KYSO) antibody medicines®. Established in 1999, Genmab is headquartered in Copenhagen, Denmark, with international presence across North America, Europe and Asia Pacific. For more information, please visit Genmab.com and follow us on LinkedIn and X. Contact:
David Freundel, Senior Director, Product CommunicationsT: +1 609 613 0504; E: dafr@genmab.com Andrew Carlsen, Vice President, Head of Investor RelationsT: +45 3377 9558; E: acn@genmab.comThis Media Release contains forward looking statements. The words “believe,” “expect,” “anticipate,” “intend” and “plan” and similar expressions identify forward looking statements. Actual results or performance may differ materially from any future results or performance expressed or implied by such statements. The important factors that could cause our actual results or performance to differ materially include, among others, risks associated with preclinical and clinical development of products, uncertainties related to the outcome and conduct of clinical trials including unforeseen safety issues, uncertainties related to product manufacturing, the lack of market acceptance of our products, our inability to manage growth, the competitive environment in relation to our business area and markets, our inability to attract and retain suitably qualified personnel, the unenforceability or lack of protection of our patents and proprietary rights, our relationships with affiliated entities, changes and developments in technology which may render our products or technologies obsolete, and other factors. For a further discussion of these risks, please refer to the risk management sections in Genmab’s most recent financial reports, which are available on www.genmab.com and the risk factors included in Genmab’s most recent Annual Report on Form 20-F and other filings with the U.S. Securities and Exchange Commission (SEC), which are available at www.sec.gov. Genmab does not undertake any obligation to update or revise forward looking statements in this Media Release nor to confirm such statements to reflect subsequent events or circumstances after the date made or in relation to actual results, unless required by law. Genmab A/S and/or its subsidiaries own the following trademarks: Genmab®; the Y-shaped Genmab logo®; Genmab in combination with the Y-shaped Genmab logo®; HuMax®; DuoBody®; HexaBody®; DuoHexaBody®, HexElect® and KYSO®. EPCORE®, EPKINLY®, TEPKINLY® and their designs are trademarks of AbbVie Biotechnology Ltd.
i Engelberts PJ, et al. DuoBody-CD3xCD20 Induces Potent T-Cell-Mediated Killing of Malignant B Cells in Preclinical Models and Provides Opportunities for Subcutaneous Dosing. EBioMedicine. 2020;52:102625. doi: 10.1016/j.ebiom.2019.102625. Media Release no. i08CVR no. 2102 3884LEI Code 529900MTJPDPE4MHJ122 Genmab A/SCarl Jacobsens Vej 302500 ValbyDenmark
Attachment
140525_MRi08_EHA Curtain Raiser
ImmunotherapyClinical ResultPhase 3Phase 2Drug Approval
04 Feb 2025
New Delhi: With more than 1.5 million active cases across the country, the prevalence of
cancer
in India has certainly outpaced the available treatment interventions. However, what makes the situation even worse is the sky-high cost of treatment, which makes these therapies inaccessible to many patients, leaving them in a desperate struggle to secure financial aid and loans for life-saving medicines.
A walk around Mumbai’s Tata Memorial Hospital, India’s premium cancer care institution, shows the appalling situation. T he entrance is always overflowing with patients from across the country, while many managing to live in makeshift shelters in extreme financial distress to cope with costs of treatment.
A study showed a small fraction of Indians, roughly 3 per cent can afford expensive immunotherapies in India, making it a big challenge for public health establishments to reach modern medicines to the low income strata.
To confront this growing crisis, the government has opted for a strategy of waiving customs duties on life-saving medications, thereby reducing import costs and hoping to translate these savings into more affordable treatment options for patients.
The Finance Minister said in her
Budget 2025
speech, “To provide relief to patients, particularly those suffering from cancer, rare diseases, and other severe chronic conditions, I propose to add 36 life-saving
drugs
and medicines to the list of medicines fully exempted from Basic
Customs Duty
.”
She also proposed to add “six life-saving medicines to the list attracting a concessional customs duty of 5 percent.”
The government order states, “In exercise of the powers conferred by sub-section (1) of section 25 of the Customs Act, 1962 (52 of 1962), the Central Government, on being satisfied that it is necessary in the public interest to do so,” has exempted Basic Customs Duty on 36 life-saving medications.
A rough analysis of the list shows that out of the 36 medications, 25 are indicated for the treatment of various types of cancer. Feb 4,
World Cancer Day
, is the perfect time to assess whether exempting customs duties could make a significant difference to patient expenses.
The majority of these drugs are protected by patents, granting them global exclusivity, and are marketed by major pharmaceutical giants such as Merck, Roche, GSK, and AstraZeneca.
Strikingly, in the previous Budget 2024, the government announced the
exemption
of customs duty on three cancer drugs—Osimertinib, Durvalumab, and Trastuzumab Deruxtecan.
The first two medications are indicated for lung cancer, while the latter is used in the treatment of breast cancer. All three are marketed by UK pharma giant AstraZeneca.
According to the government, “Drugs/medicines generally attract a basic customs duty of 10 percent, while some categories of life-saving drugs/vaccines attract a concessional rate of 5 percent or nil.”
The Basic Customs Duty (BCD) is calculated over the customs value, which is the accumulation of the CIF cost where C represents the cost of goods, I stands for insurance, and F accounts for freight or logistics costs.
Besides BCD, the government levies a social welfare surcharge of 10 per cent, calculated over the BCD value, and a 5 per cent Integrated Goods and Services Tax (IGST), calculated over the accumulated figure of CIF value and BCD of the imported goods.
Speaking to ET Pharma, Leena Menghaney, a lawyer and public health activist, stressed, “The customs duty waiver on some of the exorbitantly priced cancer and rare disease drugs is a welcome move, but it is like a band-aid and does not address the underlying problem of high prices due to patent monopolies that block local production.”
“India is a country where half the population lives on less than $1 per day and bears a large public health burden for cancers. How can patients pay for patented medicines like
Keytruda
?” she added.
While industry stakeholders are still analysing the net impact of the announcement, Dr.B. S. Ajaikumar, Executive Chairman of Healthcare Global Enterprises Limited, said, “The exemption of customs duty will have an impact of 5-10 percent on the price of drugs, and this development will primarily benefit cash-paying patients, with some benefit extending to those utilizing insurance.”
Bhanu Prakash Kalmath, a consultant at Grant Thornton Bharat LLP, underlined, “The final reduction will depend on manufacturers, hospitals, and supply chains. This move directly reduces import costs and supports broader access to essential treatments. It also benefits insurance providers, government health schemes like
Ayushman Bharat
, and Patient Assistance Programs (PAPs).”
Under the Patient Assistance Programs (PAP) offered by pharmaceutical companies, medicines are provided in 1+1 or 2+1 packages, where the patient pays for one or two drugs and receives an additional one free of cost.
However, under the condition of anonymity, an expert shared that very few patients in the country are currently covered under PAP programs. Additionally, at times, the last-stage providers (mostly hospitals) that stock these drugs apply a profit margin over the products.
Furthermore, it is crucial to note that while the Finance Minister’s announcement has exempted customs duties, patients still have to contend with the Maximum Retail Price (MRP) of drugs. The MRP is compiled based on several other costs, as post-import, the product often requires special storage, handling, and packaging before distribution to retail outlets.
The distribution costs include transportation to wholesalers and retail outlets, along with the distributor’s margin.
To make novel treatments truly affordable for the Indian demographic, Menghaney emphasised, “The country needs to boost local production and competition by granting a compulsory license clause. This would cut the cost of the drug by allowing another company to manufacture the therapy, even though it is still under patent.”
“In the past, such measures have slashed prices by introducing local generic versions of cancer drugs—by an astounding 97 percent,” she added.
The high price of cancer drugs has long been a contentious issue across low- and middle-income countries (LMICs), including India. However, given the scale and severity of the problem, it categorically requires a more holistic solution—one that ensures benefits are truly materialized for patients.
As philanthropist Bill Gates once said,
“The good news is that we have the tools to prevent most diseases. But the bad news is that not enough people are getting them.”
List of Cancer Drugs to exempted from BCD under Budget 2025-26
S. No.
Drug
Company
Brand Name
MRP (INR)
1.
Pembrolizumab
MSD (Merck, Sharp & Dohme)
Keytruda
2,16,500
2.
Lorlatinib
Pfizer
Lorbrena
10,400
3.
Dacomitinib
Pfizer
Vizimpro
2,135
4.
Inotuzumab Ozogamicin
Pfizer
Besponsa
3,30,000
5.
Ribociclib
Novartis
Kisqali
1,125
6.
Dabrafenib
Novartis
Tafinlar
1,516
7.
Selumetinib
AstraZeneca
Koselugo
-
8.
Fulvestrant
AstraZeneca
Faslodex
21,100
9.
Acalabrutinib
AstraZeneca
Calquence
15,147
10.
Olaparib
AstraZeneca
Lynparza
5,416
11.
Amivantamab
J&J
Rybrevant
98,500
12.
Teclistamab
J&J
Tecvayli
76,218
13.
Daratumumab and hyaluronidase-fih
J&J
Darzalex Faspro
2,64,600
14.
Ibrutinib
J&J
Imbruvica
3,644
15.
Bortezomib
J&J
Velcade
18,060
16.
Daratumumab
J&J
Darzalex
18,900
17.
Cetuximab
Merck Specialties (Merck KGaA)
Erbitux
21,250
18.
Avelumab
Merck Specialties
Bavencio
-
19.
Tepotinib
Merck Specialties
Tepmetko
5,094
20.
Brentuximab Vedotin
Takeda
Adcetris
2,22,709
21.
Vedolizumab
Takeda
Entyvio
-
22.
Atezolizumab
Roche
Tecentriq
2,77,708
23.
Pertuzumab+trastuzumab
Roche
Phesgo
2,80,000
24.
Polatuzumab vedotin
Roche
Polivy
2,22,225
25.
Alectinib
Roche
Alecensa
2,215
Note* the above price is without any deduction and of lowest dosage strength as some of the drugs are available in multiple strengths and their prices also very depending upon that particular strength.
By
Abhijeet Singh
,

ImmunotherapyBiosimilar
01 Feb 2025
New Delhi : Continuing its focus on addressing the challenge of skyrocketing
drug prices
, the Finance Minister, in her eighth Budget speech for the fiscal year 2025-26, announced a full exemption of basic customs duty (BCD) for 36 life-saving medications indicated for critical conditions such as cancer, autoimmune diseases, and rare disorders.
“To provide relief to patients, particularly those suffering from cancer, rare diseases, and other severe chronic conditions, I propose to add 36 lifesaving drugs and medicines to the list of medicines fully exempted from Basic Customs Duty,” the Finance Minister said in her Budget speech.
Previously, the exemption of BCD on these drugs was conditional on their availability free of cost under drug companies' Patient Assistance Programmes (PAP).
Among these 36 medications, 25 are indicated for the treatment of different types of cancer, five are prescribed for rare disorders, two for respiratory diseases, three for autoimmune conditions, and one is used in the treatment of retinal diseases.
The majority of these drugs are protected by patents, granting them global exclusivity, and are marketed by major pharmaceutical giants such as Merck, Roche, GSK, and AstraZeneca.
Notably, in the previous Budget, the government announced the exemption of customs duty on three cancer drugs—Osimertinib, Durvalumab, and Trastuzumab Deruxtecan. The first two medications are indicated for lung cancer, while the latter is used in the treatment of breast cancer patients.
Sharing his views on this announcement, Arjun Juneja, COO of Mankind Pharma, said, “The exemption of Basic Customs Duty on 36
life-saving drugs
makes critical treatments, especially for oncology and rare diseases, more affordable. It aligns with the government’s vision of a self-reliant India, lays a solid foundation for the
pharmaceutical sector
, and enhances
healthcare accessibility
.”
“The addition of 36 life-saving drugs to the list of medicines fully exempted from basic customs duty, along with six more medicines under concessional duty, will significantly reduce treatment costs for patients,” added DS Negi, CEO of Rajiv Gandhi Cancer Institute & Research Centre (RGCIRC).
Commenting on its impact on patients, Dr. Tarang Gianchandani, CEO of Sir H.N. Reliance Foundation, said, “The
basic customs duty exemption
on 36 life-saving drugs, including those for cancer and rare diseases, ensures that critical treatments remain within reach for countless patients and their families, bringing hope.”
List of 36 Medications given BCD Exemptions:
S.No.
Drug
Company
Clinical Indication
1.
Pembrolizumab
MSD (Merck, Sharp & Dohme)
Cancer
2.
Lorlatinib
Pfizer
Cancer
3.
Dacomitinib
Pfizer
Cancer
4.
Inotuzumab Ozogamicin
Pfizer
Cancer
5.
Ribociclib
Novartis
Cancer
6.
Dabrafenib
Novartis
Cancer
7.
Selumetinib
AstraZeneca
Cancer
8.
Fulvestrant
AstraZeneca
Cancer
9.
Acalabrutinib
AstraZeneca
Cancer
10.
Olaparib
AstraZeneca
Cancer
11.
Amivantamab
J&J
Cancer
12.
Teclistamab
J&J
Cancer
13.
Daratumumab and hyaluronidase-fih
J&J
Cancer
14.
Ibrutinib
J&J
Cancer
15.
Bortezomib
J&J
Cancer
16.
Daratumumab
J&J
Cancer
17.
Cetuximab
Merck Specialties (Merck KGaA)
Cancer
18.
Avelumab
Merck Specialties
Cancer
19.
Tepotinib
Merck Specialties
Cancer
20.
Brentuximab
Takeda
Cancer
21.
Vedolizumab
Takeda
Cancer
22.
Atezolizumab
Roche
Cancer
23.
Pertuzumab+trastuzumab
Roche
Cancer
24.
Polatuzumab vedotin
Roche
Cancer
25.
Alectinib
Roche
Cancer
26.
Benralizumab
Astrazeneca
Respiratory Disease (Asthma)
27.
Mepolizumab
GSK
Respiratory Disease (Severe Asthma)
28.
Ustekinumab
J&J
Autoimmune Disease (Psorasis)
29.
Ocrelizumab
Roche
Autoimmune Disease
(Multiple sclerosis)
30.
Luspatercepto
Bristol Myers Squibb
Autoimmune Disease (Anemia)
31.
Velaglucerase
Takeda
Rare Disorder
(Type 1 Gaucher disease)
32.
Agalsidase Alpha
Takeda
Rare Disorder (Fabry disease)
33.
Idursulphase
Takeda
Rare Disorder (Hunter syndrome)
34.
Risdiplam Powder
Roche
Rare Disorder (SMA)
35.
Emicizumab
Roche
Rare Disorder (Hemophillia)
36.
Faricimab
Roche
Retinal Disease
By
Online Bureau
,

100 Deals associated with Polatuzumab
Login to view more data
R&D Status
10 top R&D records. to view more data
Login
| Indication | Highest Phase | Country/Location | Organization | Date |
|---|---|---|---|---|
| Diffuse Large B-Cell Lymphoma | Clinical | - | - |
Login to view more data
Clinical Result
Clinical Result
Indication
Phase
Evaluation
View All Results
| Study | Phase | Population | Analyzed Enrollment | Group | Results | Evaluation | Publication Date |
|---|
Clinical | Diffuse Large B-Cell Lymphoma First line | 38 | Polatuzumab-R-CHP | euahrqrreg(bprcvfwadw) = blklpqvmxf eoqexzzgqc (xawjdavmwu ) View more | Positive | 14 May 2025 | |
Phase 2 | Aggressive Non-Hodgkin Lymphoma First line | 80 | Rituximab-Polatuzumab-Glofitmab (R-Pola-Glo) | oifjnkyzxy(aqqtlnechb) = bjourpoeyj dhcknoznuq (jqoznyhszf, 81.2 - 95.6) View more | Positive | 14 May 2025 | |
Not Applicable | 58 | Pola-BR | ddikdtsvfo(iectuuqgeo) = vtfmihxcil lxtcrbgqcp (njldiutxck, 1.9 - 4.1) View more | - | 08 Jun 2023 | ||
Not Applicable | Diffuse large B-cell lymphoma recurrent serum lactate dehydrogenase (LDH) | - | Polatuzumab vedotin (PV) with rituximab and bendamustine | lrnvuvhfvc(jaranoxypv) = ldemkmphmp oqcgubzqby (zcijhdthal, 14 - 21) View more | Positive | 01 Mar 2022 | |
Not Applicable | Aggressive B-Cell Non-Hodgkin Lymphoma Hemoglobin | platelet count | liver function tests ... View more | 75 | gqnkqwfwyt(xyzbqwudbz) = dsgbzstkfh fvghbvbesa (inyvmnmhak ) View more | - | 01 Mar 2022 | ||
gqnkqwfwyt(xyzbqwudbz) = taolnukljk fvghbvbesa (inyvmnmhak ) View more |
Login to view more data
Translational Medicine
Boost your research with our translational medicine data.
login
or
Deal
Boost your decision using our deal data.
login
or
Core Patent
Boost your research with our Core Patent data.
login
or
Clinical Trial
Identify the latest clinical trials across global registries.
login
or
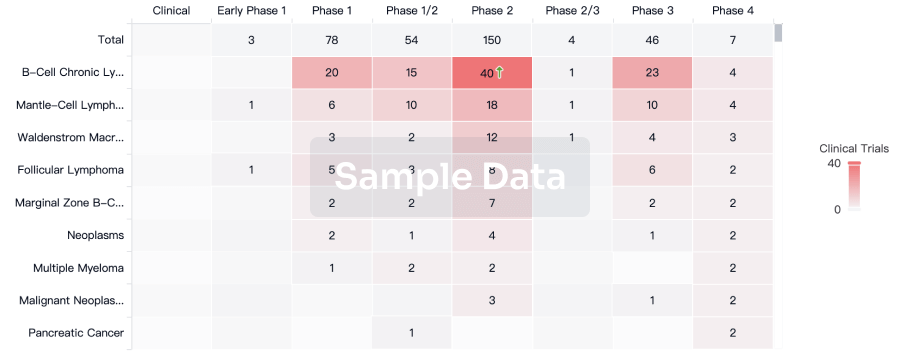
Approval
Accelerate your research with the latest regulatory approval information.
login
or
Biosimilar
Competitive landscape of biosimilars in different countries/locations. Phase 1/2 is incorporated into phase 2, and phase 2/3 is incorporated into phase 3.
login
or
Regulation
Understand key drug designations in just a few clicks with Synapse.
login
or
AI Agents Built for Biopharma Breakthroughs
Accelerate discovery. Empower decisions. Transform outcomes.
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Empower better decisions with the latest in pharmaceutical intelligence.
Bio
Bio Sequences Search & Analysis
Sign up for free
Chemical
Chemical Structures Search & Analysis
Sign up for free